Research & Innovation Application Form Guidance
The hospital has put in place a number of research application procedures to ensure that the wellbeing and rights of hospital patients and staff, and the hospital’s resources, are safeguarded at all times. Staff conducting any form of research related project are required to submit an application form to the R&I Office, to register their study and to allow further review by one of the relevant subgroups for the purposes of approval, oversight and feedback. Projects must not commence until an official approval email is received from the R&I Office.
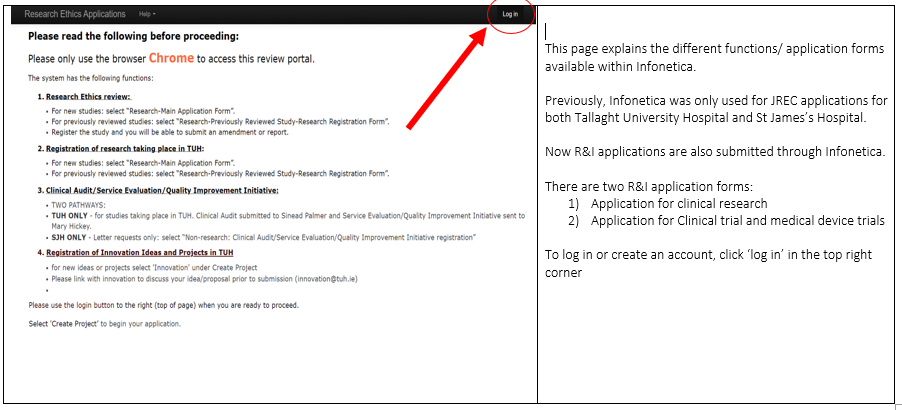
From January 1st, 2025 the R&I application process will move to the SJH/TUH Joint Research Ethics Committee (JREC) online system, which can be found at: https://sjh-tuh.forms.ethicalreviewmanager.com/
We have created a helpful Guide to assist you while completing the new R&I Application form:
R&I Application Guidance Document 2025 & R&I Application Guidance PowerPoint 2025
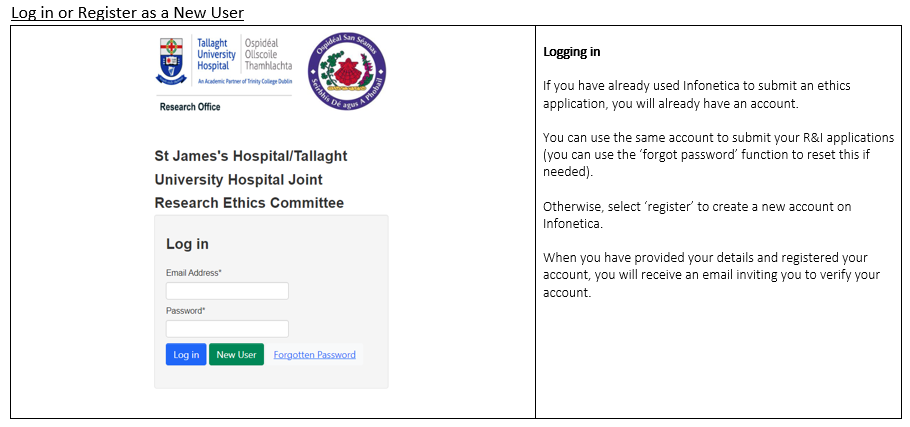
The Research & Innovation (R&I) Office are asking all Principal Investigators & researchers to create a user account on the JREC system (using the above link) before January 1st, 2025, to ensure a smooth transition. Principal Investigators & researchers who have previously completed research ethics applications to JREC will already have a user account.
New users can create accounts by following the step below;
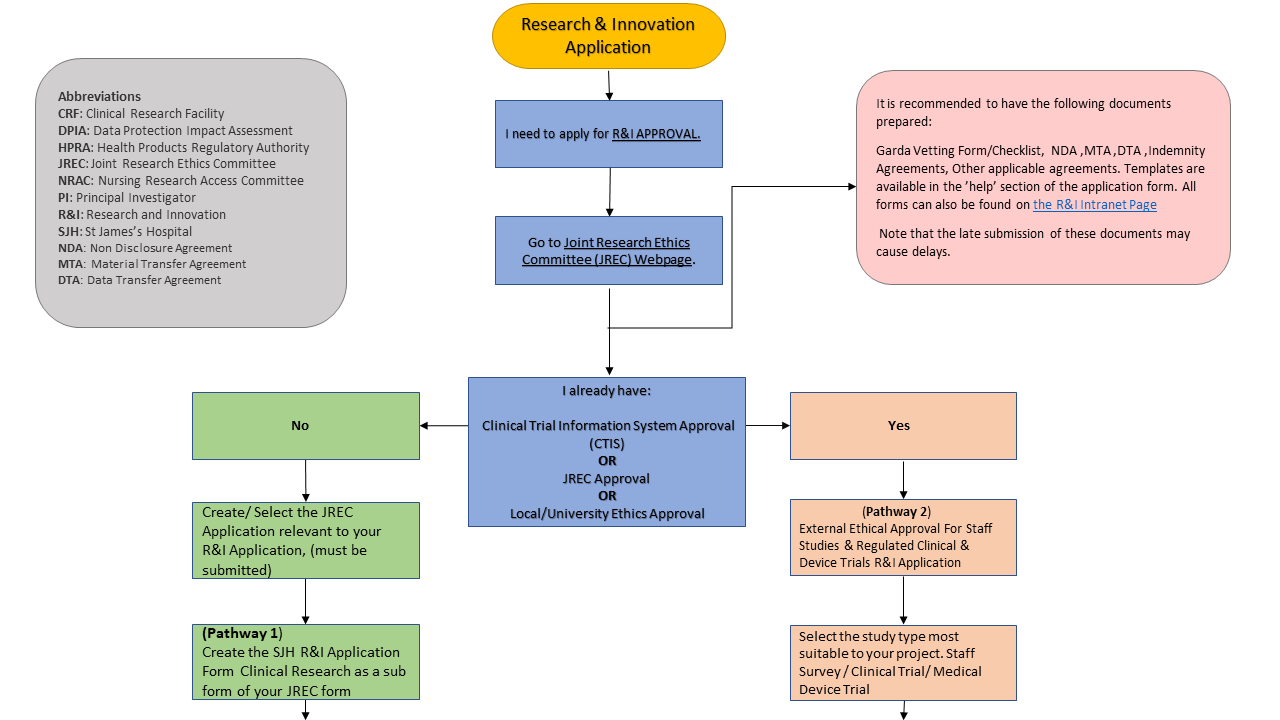
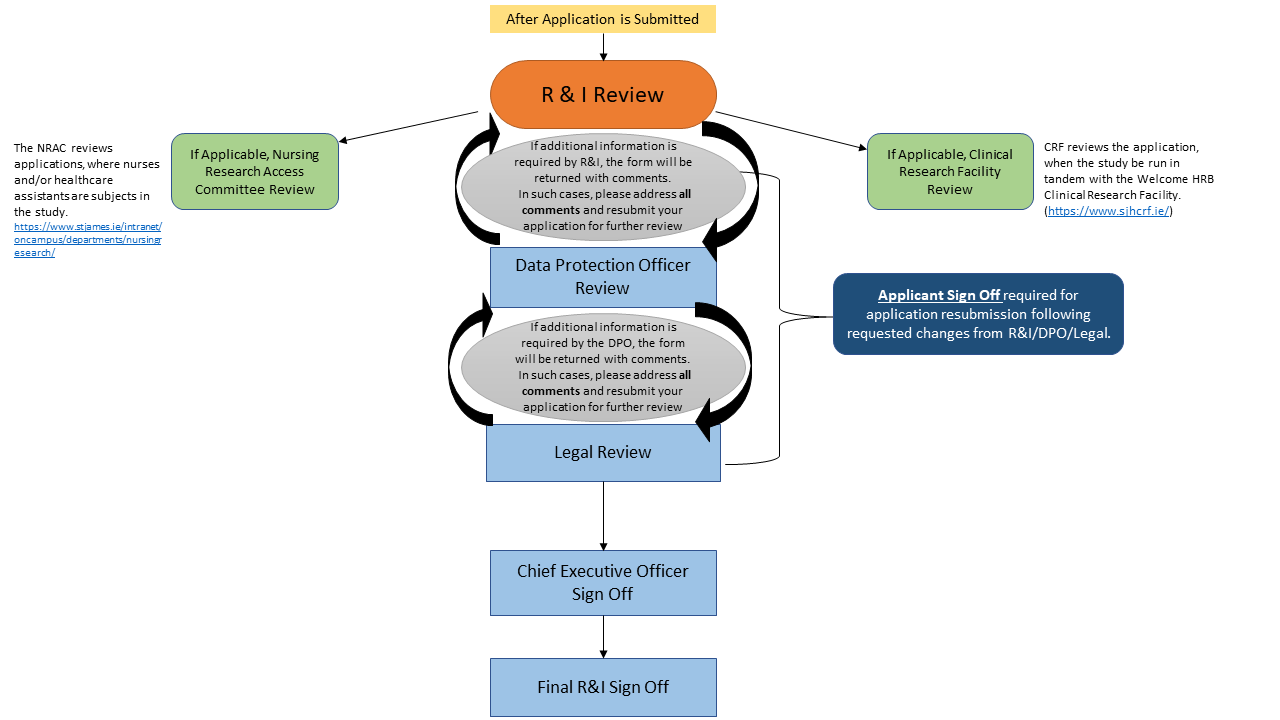
The steps for R&I approval will remain the same.
However, the benefits of integration with the JREC system are as follows:
- Streamlined approval process.
- Reduced administrative burden on researchers via:
- Single DPIA for both JREC & SJH. For clinical & medical device trials, the Sponsor DPIA remains acceptable..
- Auto population of JREC responses into the R&I application form, where questions are duplicated. - One system for both JREC & R&I to simplify the process of research approval.
- Applicants will be able to track their application’s progress throughout the approval process on the new system.
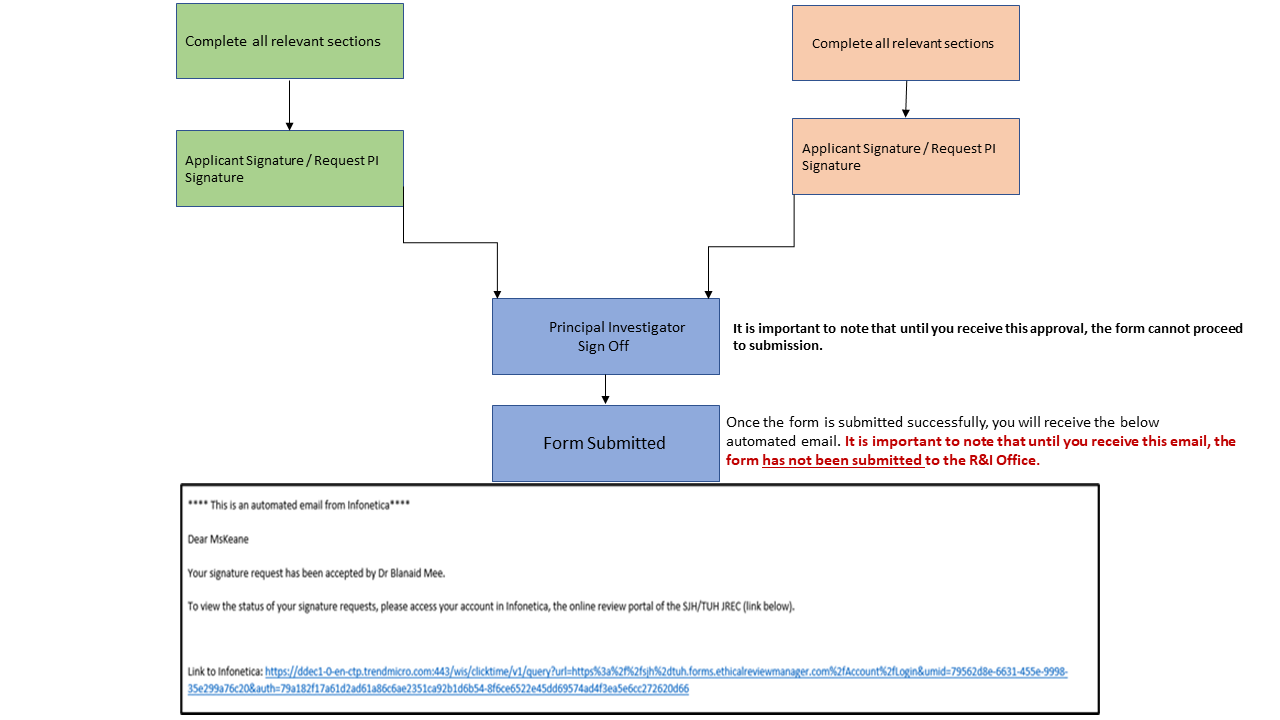
Further updates will be posted on the R&I Intranet Webpage to keep Principal Investigators and researchers informed.
Abbreviations
CRF: Clinical Research Facility
DPIA: Data Protection Impact Assessment
HPRA: Health Products Regulatory Authority
JREC: Joint Research Ethics Committee
NRAC: Nursing Research Access Committee
PI: Principal Investigator
R&I: Research and Innovation
SJH: St James’s Hospital
-
- You can access the application form via any of the following links:
- SJH R&I Office intranet page, accessible via the ‘Departments’ tab
- SJH intranet under the ‘Hospital Forms’ tab, in the ‘Resources’ section
- Under the ‘Research’ tab in the ‘Education’ section.
- A version of the form that can be accessed outside of the SJH network is available by emailing research@stjames.ie (The external version of the form cannot be saved and returned to, and must be completed inone sitting).
- Complete all required sections of the form using the free-text boxes, tick-boxes, and dropdown menus. Depending on your selections and the information you provide, additional sections will appear that will require completion. Use the information icons to access relevant information about each section.
- Once you submit the form, you will be brought to the ‘Thank You’ page, which will display your application reference number, provide you with the option to print your submission, and provide links to other related guidelines. An automatic email confirmation will also be sent.
- An email will be sent to the Principal Investigator that you selected in the form, he/she will receive an email from onlineforrms with a link to approve the application. This must be done while on the SJH network.
- Additional documentation will also be required, depending on the type of research, e.g. ethics approval, HPRA approval, study protocol, contracts, nondisclosure agreements, consent forms, patient information leaflets etc. Email all required documents to research@stjames.ie, referring to your submission reference number.
- The application will be registered with the R&I Office, screened, and forwarded to the appropriate sub-group for review, including the Clinical Research Facility (CRF), the Nursing Research Access Committee (NRAC), the Patient Survey/ Focus Group Advisory Panel, the Legal Office, or the Deputy CEO. Fully completed submissions, which include associated documentation, are reviewed by the CRF weekly, and by the NRAC on a monthly basis. Draft contracts will be reviewed by the Legal Office, and agreed contracts will be signed by the DCEO within 7 working days.
- A submission can be made in parallel with an Ethics Committee and HPRA submission (if required). Please forward confirmation of Ethics Committee and HPRA approval (as applicable) to the R&I Office when received, without which the approval process cannot proceed.
- Hospital approval will be confirmed by the R&I Office upon satisfactory review, and the study cannot proceed until an approval email has been issued. Version 7, October 2019 3
- You may be contacted to clarify the information submitted, to provide additional supporting information, or to address any omissions.
- Annual progress report summaries are required to be submitted to the R&I Office (research@stjames.ie), in addition to an end-of-trial report upon completion of the study. The update element required and questions include:
- Did the study begin / take place?
- If yes above, has the collection of data been concluded?
- Incidents / issues arising of note (if any)?
- What dissemination of findings has been done / is planned?
- Has the ethics committee been provided with an annual update?
- Did the HPRA inspect the study?
- If yes, what were the main findings?
- You can access the application form via any of the following links:
-
The PI is the lead researcher and the individual responsible for the on-site conduct of the study, and the team lead when the study is conducted by a research team. The PI has overall responsibility for all aspects of the study, and should be a hospital consultant for large and high-risk studies (e.g. clinical trials, medical device trials, funded studies and clinical research at the level of Masters or above), to ensure the activation of indemnity cover. Smaller studies can be run by researchers with the approval of local managers, in addition to R&I Office approval. It is recommended that you add your line manager in the Principal Investigator/Supervisor section so that they can approve the application form. External researchers require a local hospital based PI collaborator or supervisor to conduct patient related research in the hospital. NRAC applicants will require a “gatekeeper” to facilitate local logistics.
-
External research personnel will need to be suitably qualified, the study that they are involved with will need to be under the direction of a suitable PI, they will require the supervision of an appointed hospital staff member, and agreements will need to be place, including a non-disclosure agreement. For external research personnel who plan to come on-site and to have direct patient contact, they will also need Garda Vetting confirmation (from organisations that the hospital has an information exchange agreement in place with), or a full Garda Vetting submission. There are two Garda Vetting forms to be completed (available
from the R&I Office), which request information including, full name, date of birth, current address (including Eircode), email address, contact number, and the role being vetted for.The documents must be presented to the R&I Office along with two suitable forms of ID, which will be validated and photocopied, and sent to the hospital Human Resources Department. Processing takes at least 10 days. In addition, mandatory trainingwill have to be completed via HSELand, including Children First and Clinical Hand Hygiene training, with certificates of completion sent to the R&I Office.
-
If the purpose of your project is to generate new knowledge then this indicates that you are conducting research. Findings that can be generalised to other organisations is another feature of research therefore will require an application to an Ethics Committee from participants. The research could involve an intervention or could involve data analysis only. Either way, the research will require an application to an Ethics Committee from participants.
From 6th August 2024, Quality, Safety and Improvement Directorate (QSID) will be reviewing non-research studies such as Clinical Audit, Service Evaluation or Usual Practice Study. Applicants for these studies are asked to register with QSID by completing a Clinical Effectiveness Study Form on the Hospital’s Quality and Safety Information Management System.
You can access this registration system via the ‘useful links’ tab on the Hospital Intranet Page.
If you remain unsure if your project is ‘research’ or ‘non-research’, we have a helpful guide: Is my project “research” or “non-research”?
If you have any further questions, please contact research@stjames.ie
-
- Clinical Trials (Drug Trials)
- Clinical Research Study
- Staff Study
- Patient Survey / Focus Group
- Medical Device Trial
-
- Patient based research requires ethical approval and explicitly informed patient consent. For research based in SJH, ethics submissions should be made to the local ethics committee, which is the Tallaght Hospital / St James's Hospital JREC.
- Details on the JREC Ethics submission process, including the JREC application form can be found on the Tallaght/SJH Joint Research Committee website:Tallaght/SJH Joint Research Committee website
- For clinical trials and the national Covid 19 biobank, ethics applications can be made to National Research Ethics Committees (NRECs) whose remit is to deliver a single national ethics opinion for regulated health research studies involving the following;
o clinical trials on medicinal products
o clinical investigations of medical devices
o performance studies of in vitro diagnostic medical devices
o national Irish COVID19 biobank.
Further information on the NREC can be found here: https://www.nrecoffice.ie/about/national-office/- Research projects will not be covered by insurance unless ethical approval is in place.
-
- Informed consent is required for any form of interventional health research.
- Retrospective chart review research does not require explicit consent if it is deemed to be low risk and conducted by an SJH employee or student healthcare practitioner under the supervision of an SJH employee. Ethics approval is required.
- Please see the Department of Health Guidelines on the informed consent process:
- The Tallaght/SJH JREC website also has a template patient consent form and patient information leaflet on their website.
-
St James’s Hospital is committed to the provision of a safe and secure environment for patients and staff, and embraces its obligation to protect individuals' rights to privacy and confidentiality. Individuals conducting research must comply with the SJH data protection policies and national regulation.
A DPIA must be completed for any projects that involve processing personal data.
You can access the DPIA template in the ‘forms and booklets’ section of the R&I intranet page. Please send the completed form to research@stjames.ie. Remember when completing the form that your focus should be on how the data is collected, used, stored etc.Researchers who obtain or process personal information must ensure that they follow the Principles of Data Protection:
- Obtain and process the information fairly.
- Keep it only for one or more specified and lawful purposes.
- Process it only in ways compatible with the purposes for which it was given
to you. - Keep it safe and secure.
- Keep it accurate and up-to-date.
- Ensure that it is adequate, relevant and not excessive.
- Retain it no longer than is necessary for the specified purpose or purposes.
- Provide a copy of his/her personal data to any individual, on request.
The safeguarding of research data is managed by the Principal Investigator,
operating on behalf of the Data Controller, who ensures that:- Researchers apply for permission to access information for research purposes.
- Suitable patient consent is in place.
- Research team members are sufficiently informed about data protection
obligations. - Data protection related risks have been considered and mitigated.
- Research data is saved in secure folders on the shared drive, and not stored
on the hard disk of a PC, and that paper based records are stored in locked
filing cabinets. - No patient or employee data, images, or other forms of identifiable
information are stored on portable storage devices (e.g. memory stick, tablet,
laptop, smartphone), transferred by email or other electronic methods, or are
accessible to third parties without agreement. - Research data collected on-site is anonymised or pseudonymised (removing
name, Medical Record Number, and other distinguishing information),
especially if being taken off site, and if coding is used, the key to re-identify
coded data remains on-site and is held by the PI.